«Work, work, and work harder»
With the life-saving drug CAL02, Combioxin SA is revolutionizing the field of severe infections such as pneumonia. Managing Director Samareh Azeredo da Silveira Lajaunias explains how the spin-off successfully transforms the invention made at the Institute of Anatomy, University of Bern, to a market-ready pharmaceutical product.

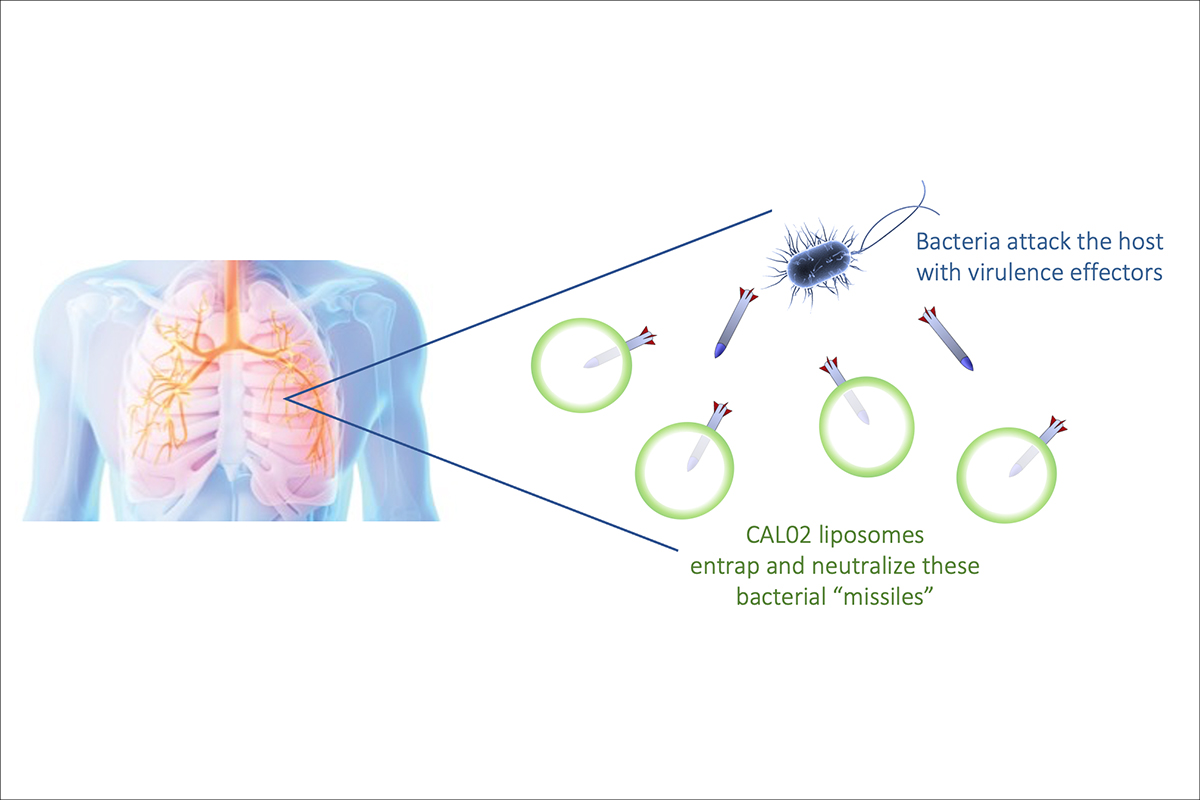
Samareh Azeredo da Silveira Lajaunias: Combioxin is a biotechnology company that develops critical care medicines for severe and fatal infections. Our lead drug candidate, CAL02, is a first-in-class drug with a novel technology and proven safety for humans. CAL02’s patented technology deploys re-engineered liposomes – tiny fat beads – to capture and neutralize bacterial toxins called virulence effectors. Traditional treatments of infections wage chemical warfare, seeking to destroy the pathogens, most frequently after dangerous toxins have already been released, destroying cells, damaging vital organs, disabling the immune system, and they trigger a potentially fatal inflammatory response in the patient.
Our recent clinical results have shown that pneumonia patients treated with CAL02 – they are put on a drip containing the drug – have a less severe disease, recover faster, and leave the intensive care unit a week earlier than patients treated only with traditional standard-of-care therapies like antibiotics. CAL02 provides a dramatically improved therapy for severe infections.
Pneumonia represents the second most frequent cause of hospitalization, the most common infection requiring admission to the intensive care unit and often results in costly and lengthy care. CAL02 allows patients to recover more rapidly and leads to a faster release from the intensive care unit and from the hospital, setting patients free from long-term follow-ups. This new drug will therefore reduce the economic burden of the disease and provide a global economic benefit.
Can you explain how CAL02 exactly works?When bacteria enter our body, they produce toxins that cause widespread damage to our tissues and organs, trigger inflammatory responses, and attack and destroy our immune cells before these can do their job, which would be to eliminate bacteria. CAL02 functions as a bait: instead of pouncing on our immune defense system and causing organ failure and inflammation, toxins are entrapped and neutralized by the CAL02 liposomes. The immune cells then have free rein and can combat bacteria and break them down. This approach changes fundamentally the fight against severe infections: since the drug does not come into direct contact with bacteria, it does not elicit the emergence of resistances as opposed to the mode of action of antibiotics which ineluctably lead to new resistances.
The relationship between Combioxin and the University of Bern lies on two pillars. One is the excellent quality of the work carried out by the technology transfer office, Unitectra, which led to an exclusive license on the intellectual property generated at the university lab and to a constructive association throughout the years. The other pillar is the trustful and positive collaboration with the inventors, the late Professor Annette Draeger and Dr. Edik Babiychuk from the Institute of Anatomy at the University of Bern. Their knowledge and expertise have been the backbone of the technology our company is developing.
What attracted you to self-employment?Several elements have motivated the leap from academic research to an entrepreneurial venture: the determination to build upon lab results and develop them into medical tools helping millions of patients, as well as the interest to explore the many other facets of an invention, including intellectual property, manufacturing, regulatory requirements, clinical development, and also marketing.

In order to move from the idea of the company to actually founding it, we initially focused on intellectual property, drug candidate formulation, efficacy confirmation and the manufacturing process. We were contacted by the technology transfer office Unitectra about Professor Draeger and Dr Babiychuk’s invention and started a collaboration with the university lab in order to confirm their initial results and refine the drug compound’s profile. Combioxin’s founders injected the necessary funds to bring the invention to clinical stage and used their expertise and network to carry out all the operational scientific, medical, manufacturing, regulatory, intellectual property, and commercial tasks for the development of the product.
What is your vision?University researchers and hospital practitioners may come up with the most innovative concepts and the most ingenious inventions. Development steps for these gems to become applicable medical tools are yet to be devised. But mainly unprecedented approaches will truly bring about life-changing medical progress. In the case of Combioxin, the invention made at the University of Bern now appears as an approach revolutionizing the field of severe infections such as pneumonia. Pneumonia is the deadliest infectious disease with still as many complications and fatal cases as decades ago, and with mortality rates for severely-ill patients as high as 30 to 50 percent despite best antibiotics available. CAL02 has been declared a medical breakthrough by The Lancet Infectious Diseases journal. The novel strategy of CAL02 is to neutralize bacterial virulence, leaving bacteria like soldiers without weapons. Designed to fill a medical gap, CAL02 can be administered to millions of patients annually, alone or in combination therapy, without adding to the resistance burden. Following positive meetings with the European and US regulatory agencies, we are setting about an ambitious clinical phase assessing CAL02 as first-line empiric therapy for complications associated with severe pneumonia, and expect the product to be approved within a timeframe of 4 years from now.
Which advice would you give your student self from todays’ perspective?An important lesson I have learned throughout the years is to properly gauge and manage the balance between two extremely different worlds: the academic labs and the pharmaceutical industry. Scientific exploration carried out in university labs reflects the magnificence of research and may lead to groundbreaking findings and inventions. It may however often clash with the focus and the consistency required for the multiple highly regulated development steps – be they pre-clinical, clinical, manufacturing, or regulatory – necessary for the invention to bloom into a market-ready pharmaceutical product.
Which advice would you give to students who are considering taking the step into self-employment?Follow your dreams, have trust in your competence, be rigorous, assess your results with an objective eye. But most of all: work, work, and work harder.
SPIN-OFFS
A spin-off is understood as the spin-off of a business unit of a company and the subsequent establishment of an independent company with this business unit. In the university environment, spin-offs are understood to be companies founded by university staff and based on research carried out at the university.
UNIAKTUELL SERIES ON SPIN-OFFS
In a loose series, the online magazine "uniaktuell" portrays spin-offs that have emerged from the University of Bern. The aim is to show how knowledge is transferred from the university into practice. Have you also realized research results at the University of Bern and dared to take the step into independence with a corresponding spin-off foundation? Contact us at uniaktuell@unibe.ch so that we can also present your company.
Unitectra
Unitectra is the technology transfer organization of the Universities of Basel, Bern and Zurich. With its services, it supports scientists in their collaborations with private industry and other public or private research institutions. The transfer of research results into new products and services is fostered in collaboration with the scientists. The transfer occurs either in collaboration with established companies or through the creation of new spin-off companies. Unitectras’ services are also available to scientists positioned at hospitals and research institutions that are associated with the three universities. Moreover, Unitectra has cooperation agreements with a number of partner institutions.
About the person

Dr. Samareh Azeredo da Silveira Lajaunias co-founded LASCCO SA in 2007 to select and launch fundamentally innovative academic discoveries for medical use. She established the company’s portfolio focusing on discoveries in the fields of critical care and infectious diseases. From 2011 to 2019, she was a partner at Sepstone Diagnostics, a spin-off of LASCCO dedicated to the development of novel biomarkers for sepsis. Since 2015, she is General Manager at Combioxin SA and is responsible for the development of CAL02, the company’s lead product. Samareh holds a Ph.D. in Neurosciences from the EPFL. She is the author of numerous publications and opinion reviews, and was designed one of the 100 outstanding personalities contributing to the dynamism and the innovation spirit in Switzerland.
About the author
Lisa Fankhauser is editor at the University of Bern Communication & Marketing Office.