"Our efforts are devoted to convert cancer from a lethal to a chronic disease"
Marianna Kruithof-de Julio and her team are developing methods to grow small clusters of cells from tumor biopsies. In this way, the researchers hope to customize treatment for each individual patient. Their goal is perhaps still five to ten years away, the expert estimates.

Marianna Kruithof-de Julio: e are developing methods to grow and multiply cells from biological samples taken from cancer patients. In doing so, we try to create favorable conditions for the cells so that they join together to form spherical cell clusters - so-called organoids. These organoids are a fairly accurate representation of the tumor from which they were derived, this cannot be achieved by conventional cell line models. Currently, we are optimizing and standardizing the procedures so that we can use the patient-specific organoids in broader tests.
What for?To be able to test which drugs are most effective for each individual patient. At the moment we have tested patient derived organoids in primary and advanced cancer stages, after the course of standard of care. My vision is that organoid testing would be incorporated in the initial clinical decision making, favored by the collaboration between the clinics and research laboratories. Pathological evaluation and organoid testing would be carried out in parallel, to identify the most promising personalized therapy.
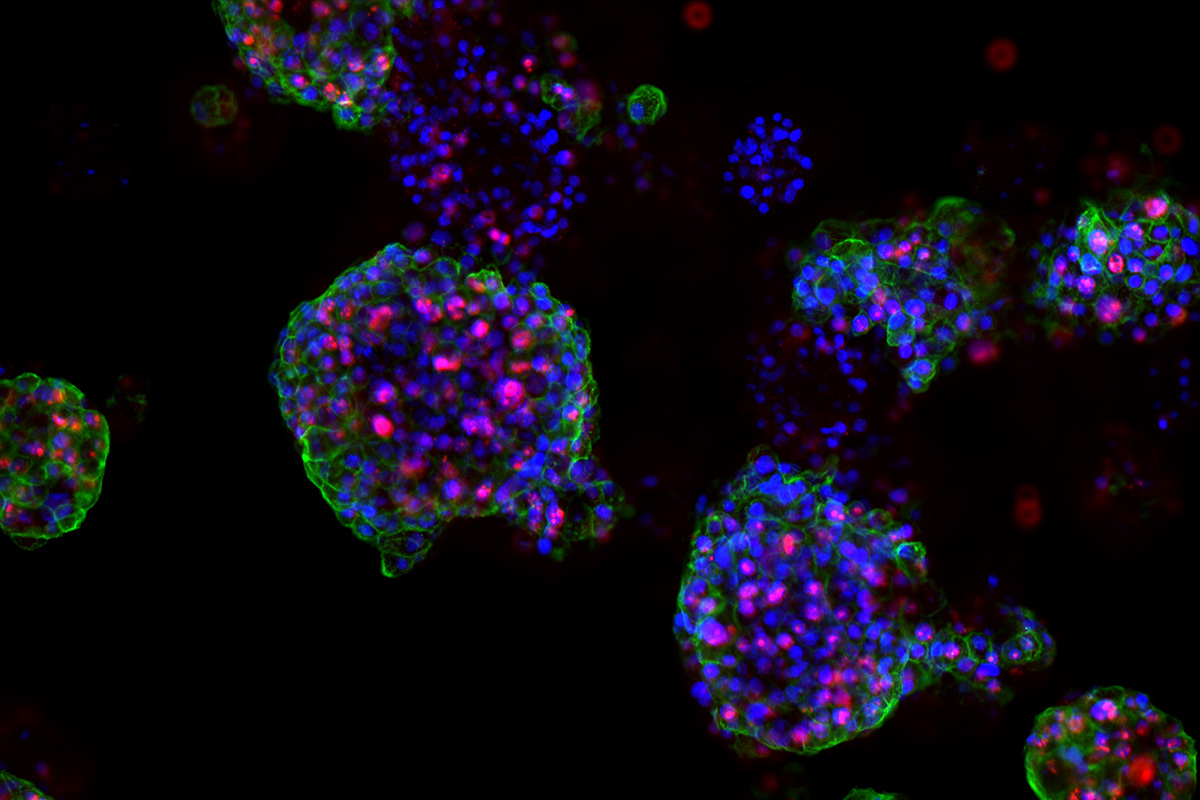
Yes, we have setup a routine platform for the derivation and testing of patient-derived organoids. Prof. George Thalmann, MD, and his colleagues at the University Department of Urology at the Inselspital in Bern, have played a crucial role in this process. In our paper we have used prostate cancer specimens to derive a novel experimental mouse model, which has allowed us to setup a medium throughput screen with FDA-approved drugs. This has resulted in the selection of 13 drugs which we then tested on patient-derived organoids. In this proof of principle approach, we have used patient-derived organoids, 2 primary and 3 advanced cases. Interestingly, from the drug testing, we could discriminate among primary non-treated versus advanced therapy resistant cases in terms of drug response. Larger cohorts and patient follow-up are needed to make our findings translatable to clinical practice which makes it challenging for prostate cancer.
Our efforts are devoted to convert cancer from a lethal to a chronic disease, which can be solely achieved by the collaboration between the researchers and the clinicians.
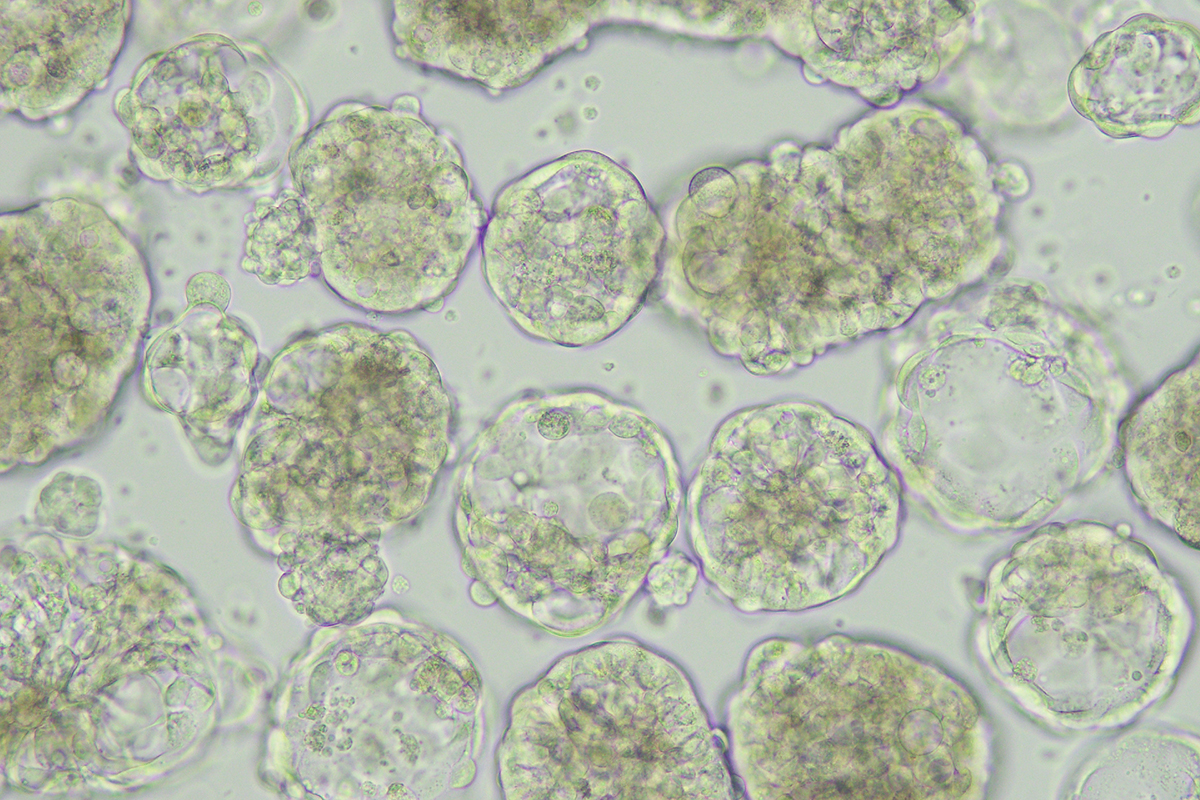
Organoids allow the study of biological processes, such as cell behavior, tissue repair and response to drugs or gene mutations, in an environment that mimics endogenous cell organization and organ structures. Starting as a major technological breakthrough they are now firmly established as an essential tool in biological research and also have important implications for clinical use. As evident from our study, organoids allow for high and medium throughput screen of drugs already in use for other cancer types, resulting in drug repurposing bypassing the need of additional animal experiments. Major advantages include that they can be grown from a limited supply of starting material, e.g. biopsies; the rapid growth compared to PDXs (patient-derived xenografts) where tissue biopsies from patients are transferred into an animal model - in this case mice. Other advantages include renewal resources for biomedical research; the potential to model 3D growth, the ability to be manipulated with gene editing tools (e.g., CRISPR-Cas9) to create model systems with similar complexity as experienced in a population of cancer patients; the direct application to precision oncology management to anticipate next or best therapies – all this will lead to a significant reduction of animal experimentation.
Media release
About Marianna Kruithof-de Julio

PD Dr. Marianna Kruithof-de Julio has been a research group leader at the Department for BioMedical Research (DBMR) and Inselspital since 2016 and member of the Bern Center for Precision Medicine (BCPM) of the University of Bern. Her main research is on the development and application of tools for precision medicine. In recent years, she has obtained several highly competitive grants, such as the USA Congressionally Directed Medical Research Program (CDMRP) and the Swiss 3R Competence Center (3RCC).
Contact:
PD Dr. Marianna Kruithof-de Julio
Urology Research Laboratory at the Department for BioMedical Research (DBMR) and Bern Center for Precision Medicine (BCPM), University of Bern and University Department of Urology at the Inselspital in Bern
Phone: +41 31 632 09 31 / marianna.kruithofdejulio@dbmr.unibe.ch
About the author
Ori Schipper is a sciene writer living and working in Bern.